If bioaerosols play any significant role in initiating rainfall it must be by triggering the Hallett-Mossop process of ice multiplication. But testing this link is difficult because there still seems to be much uncertainty about what the mechanism actually is, what triggers it and how prevalent it is.
In temperate climates rain is initiated by ice nucleation (in thunderstorms it is a mix of this and droplet coalescence). But ice nuclei are rare. Typically there are 0.1-20/L active at -15°C or below (Möhler et al. 2007), and in clean air such as over the Southern Ocean or the Australian deserts there may be only ~0.01/L at -15°C (Bigg 1973). Ice nuclei active above -10°C are very scarce.
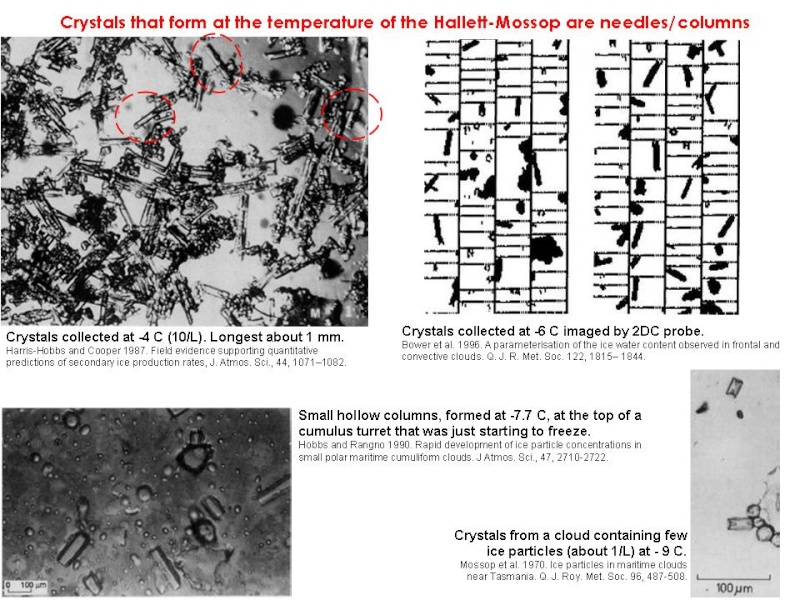
Convective clouds often rain when they shouldn’t; that is, even at their tops they are not cold enough for more than a few ice nuclei to be active (ie, -5°C to -10°C). However, in only 5-10 minutes the number of ice particles can suddenly surge by several orders of magnitude (Hallett et al. 1978, Harris-Hobbs and Cooper 1987, Mossop et al. 1970), initiating rain. For example, Hobbs and Rangno (1990) observed maritime cumulus go from no detectable ice to >350 crystals per L in 9 minutes even though no colder than -8°C. In another turret they recorded prodigious ice development in only 4 minutes between two passes at the -5.5°C level. Copious production of secondary ice particles has also been recorded in frontal clouds (Bower et al. 1996, Phillips et al. 2003).
The process was first reproduced in the laboratory by Hallett and Mossop (1974) and Mossop and Hallett (1974), who also circumscribed many of the conditions for its occurrence. Subsequent research has investigated the microscopic mechanism itself and defined the relationship between key parameters and the rate of production of ice. Saunders and Hosseini’s (2001) introduction nicely reviews this work.
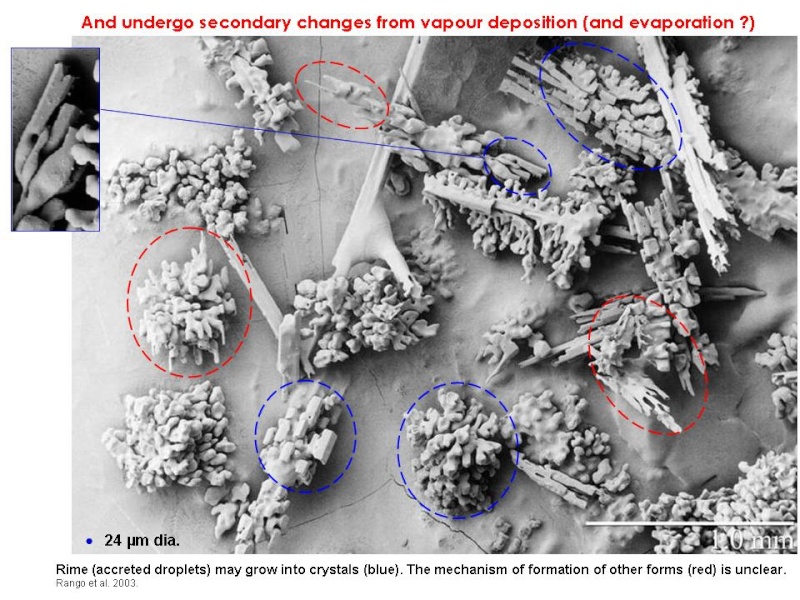
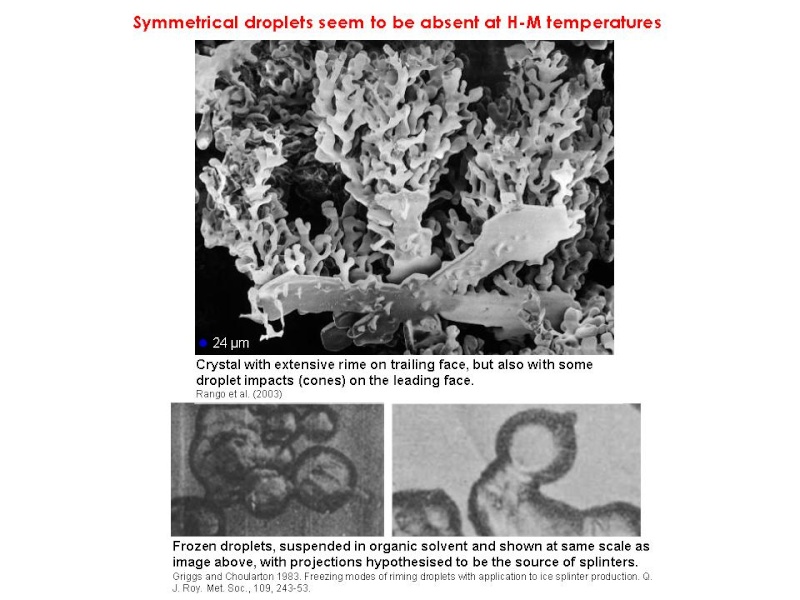
The current generally accepted hypothesis seems to be that, initially, coalescence produces small supercooled raindrops (300-500 µm) which freeze and then, by colliding with droplets, become cloaked in a filigree-like coating of rime. When this piece of graupel, up to 1-2 mm wide, hits larger droplets they may eject ice shards if they freeze onto it symmetrically from the outside-in (Griggs and Choularton 1983). These ice shards grow into needles or columns by vapour deposition to form precipitation, and possibly also more ice-particle-generating graupel. Ice production only occurs at between -3°C and -8°C (with a pronounced peak in the middle of the range) and in the presence of both large (>24 µm) and small droplets. Moderate updrafts help by keeping ice-producing particles within the zone of maximum production.
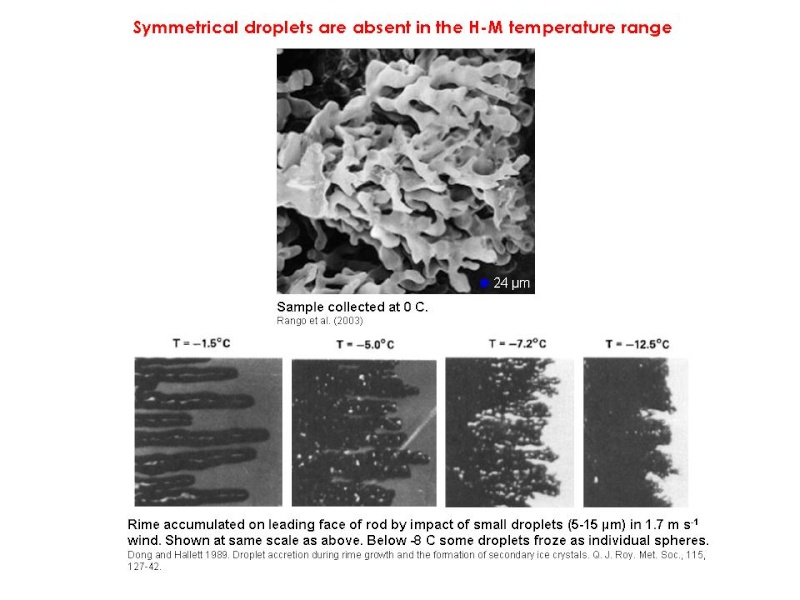
However, there is conjecture about how the splinters are produced. Dong and Hallett (1989) found that accreting droplets spread out on the ice surface, freezing as cones (frozen droplets formed only at -8°C and below). The rime that grew in their wind tunnel tests was shaped like fingers or staghorn coral. They thus thought that splinters ejected as a result of pressure build-up inside frozen accreted droplets was unlikely.
In general, Phillips et al. (2001) state that “more laboratory work is urgently required in order to establish more quantitatively, accurately and comprehensively than our present knowledge: (1) how the Hallett-Mossop process depends upon temperature, droplet size distribution and velocity of impact; and (2) how many splinters are ejected per freezing raindrop, as a function of drop size and air temperature.”
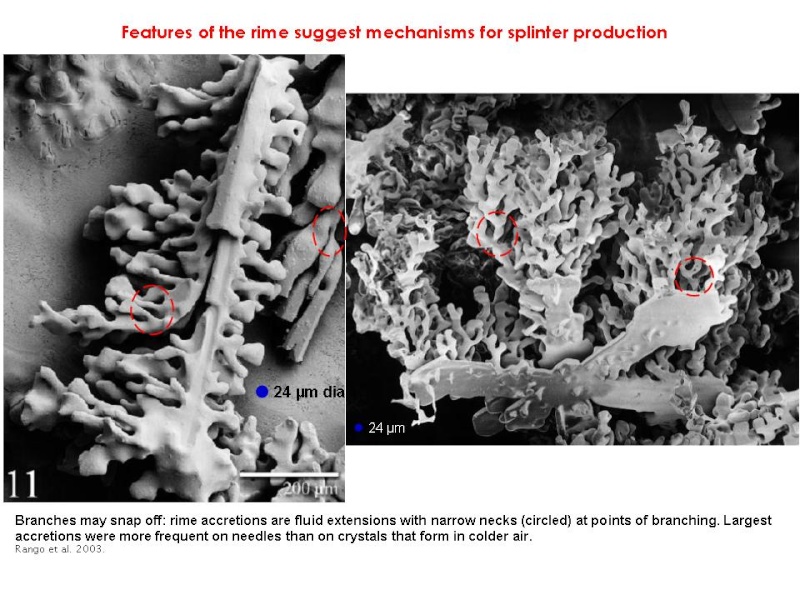
Indeed a recent and fascinating SEM analysis of graupel and its rime also found no evidence of symmetrical droplets at the appropriate temperatures (Rango et al. 2003). The structure of the graupel suggests a variety of other possible mechanisms, as shown below.





More high resolution photos of graupel and rime can be seen at the Beltsville Agricultural Research Center's Electron Microscopy Unit Snow Page:
http://emu.arsusda.gov/snowsite/rimegraupel/rg.html
http://emu.arsusda.gov/snowsite/FSSP9/FSSP9.html
Mechanisms by which these rime-covered particles might shed ice
1. Large droplets are less deviated by air flow and strike the edge of the graupel. At high temperatures they freeze more slowly (release of latent heat), and backward from the point of contact, as the tail flails in the turbulence. This progressive freezing forms the sinuous, pendulous projections. Due to a combination of lack of deflection leading to collision and release of latent heat slowing the process of freezing, larger droplets break apart, leaving behind a small freezing droplet in the wake of the graupel.
2. Large droplets strike the edge of the graupel, destabilizing its fall and initiating a tumble, causing fragile appendages to snap off.
3. Large droplets embed themselves within the open branch structure of larger graupel, attaching across several projections. When they freeze they expand and break the structure.
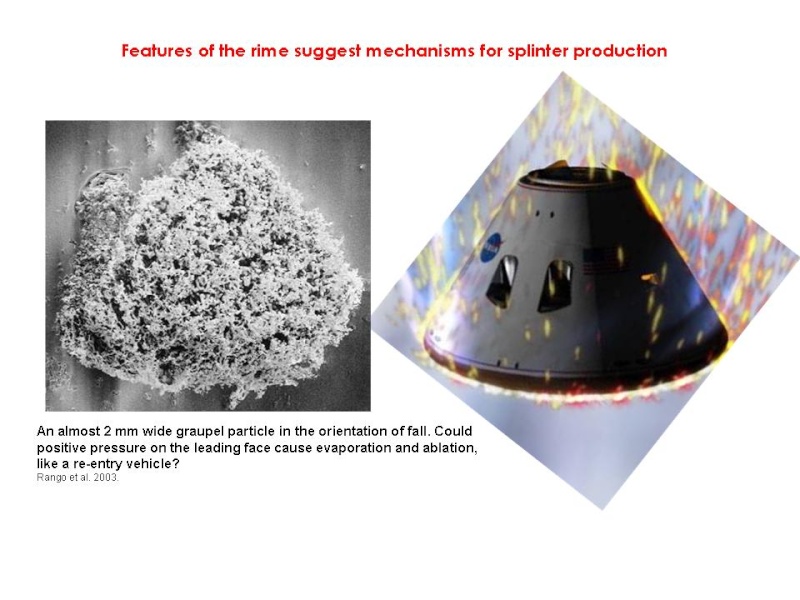
4. Branches on the leading face may evaporate as the graupel falls (below -3°C crystals only evaporate, and when evaporating branches thin to <10 µm first; Oraltay and Hallett 1989). Drier air could occur in pockets of the cloud or the cloud mass descends. Or the pressure wave in front of larger graupel cause evaporation (a 500 µm rain drop falling at 4 m/s has a 9 Pa pressure wave in front of it; Chen 2001)?
Bigg, E. K. 1973. Ice nucleus measurements in remote areas. Journal of the Atmospheric Sciences, 30, 1153–1157.
Bower, K. N., Moss, S. J., Johnson, D. W., Choularton, T. W., Latham, J., Brown, P. R. A., Blyth, A. M. and Cardwell, J. 1996. A parameterisation of the ice water content observed in frontal and convective clouds. Quarterly Journal of the Royal Meteorological Society, 122, 1815–1844.
Chen, W. H. 2001. Dynamics of sulfur dioxide absorption in a raindrop falling at terminal velocity. Atmospheric Environment, 35, 4777–4790.
Dong, Y. Y. and Hallett, J. 1989. Droplet accretion during rime growth and the formation of secondary ice crystals. Quarterly Journal of the Royal Meteorological Society, 115, 127–42.
Griggs, D. J. and Choularton, T. W. 1983. Freezing modes of riming droplets with application to ice splinter production. Quarterly Journal of the Royal Meteorological Society, 109, 243–53.
Hallett, J. and Mossop, S. C. 1974. Production of secondary ice particles during the riming process. Nature, 249, 26–28.
Hallett, J., Sax, R. I., Lamb, D. and Ramachandra Murty, A. S. 1978. Aircraft measurements of ice in Florida cumuli. Quarterly Journal of the Royal Meteorological Society, 104, 631–651.
Harris-Hobbs, R. L. and Cooper, W. A. 1987. Field evidence supporting quantitative predictions of secondary ice production rates, Journal of the Atmospheric Sciences, 44, 1071–1082.
Hobbs, P. V. and Rangno, A. L. 1990. Rapid development of ice particle concentrations in small polar maritime cumuliform clouds. Journal of the Atmospheric Sciences, 47, 2710–2722.
Möhler. O., DeMott, P.J., Vali, G. and Levin, Z. 2007. Microbiology and atmospheric processes: the role of biological particles in cloud physics, Biogeosciences, 4, 1059–1071.
Mossop, S. C., Ono, A. and Wishart, E. R. 1970. Ice particles in maritime clouds near Tasmania. Quarterly Journal of the Royal Meteorological Society, 96, 487–508.
Mossop, S.C. and Hallett, J., 1974. Ice crystal concentration in cumulus clouds: influence of the drop spectrum. Science 186, 632–634.
Oraltay, R. G. and Hallett, J. 1989: Evaporation and melting of ice crystals: A laboratory study. Atmospheric research, 24, 169–189.
Phillips, V. T. J., Blyth, A. M., Choularton, T. W., Brown, P. R. A., and Latham, J. 2001. The glaciation of a cumulus cloud over New Mexico, Quarterly Journal of the Royal Meteorological Society, 127, 1513–1534.
Phillips, V. T. J., Choularton, T. W., Illingworth, A. J., Hogan, R. J. and Field, P. R. 2003. Simulations of the glaciation of a frontal mixed-phase cloud with Explicit Microphysics Model. Quarterly Journal of the Royal Meteorological Society, 129, 1351–1371.
Rango, A., Foster, J., Josberger, E. G., Erbe, E. F., Pooley, C and Wergin, W. P. 2003. Rime and graupel: description and characterization as revealed by low-temperature scanning electron microscopy. Scanning, 25, 121–131.
Saunders, C. P. R. and Hosseini, A. S. 2001. A laboratory study of the effect of velocity on Hallett–Mossop ice crystal multiplication. Atmospheric Research 59-60, 3–14.
In temperate climates rain is initiated by ice nucleation (in thunderstorms it is a mix of this and droplet coalescence). But ice nuclei are rare. Typically there are 0.1-20/L active at -15°C or below (Möhler et al. 2007), and in clean air such as over the Southern Ocean or the Australian deserts there may be only ~0.01/L at -15°C (Bigg 1973). Ice nuclei active above -10°C are very scarce.
Convective clouds often rain when they shouldn’t; that is, even at their tops they are not cold enough for more than a few ice nuclei to be active (ie, -5°C to -10°C). However, in only 5-10 minutes the number of ice particles can suddenly surge by several orders of magnitude (Hallett et al. 1978, Harris-Hobbs and Cooper 1987, Mossop et al. 1970), initiating rain. For example, Hobbs and Rangno (1990) observed maritime cumulus go from no detectable ice to >350 crystals per L in 9 minutes even though no colder than -8°C. In another turret they recorded prodigious ice development in only 4 minutes between two passes at the -5.5°C level. Copious production of secondary ice particles has also been recorded in frontal clouds (Bower et al. 1996, Phillips et al. 2003).
The process was first reproduced in the laboratory by Hallett and Mossop (1974) and Mossop and Hallett (1974), who also circumscribed many of the conditions for its occurrence. Subsequent research has investigated the microscopic mechanism itself and defined the relationship between key parameters and the rate of production of ice. Saunders and Hosseini’s (2001) introduction nicely reviews this work.
The current generally accepted hypothesis seems to be that, initially, coalescence produces small supercooled raindrops (300-500 µm) which freeze and then, by colliding with droplets, become cloaked in a filigree-like coating of rime. When this piece of graupel, up to 1-2 mm wide, hits larger droplets they may eject ice shards if they freeze onto it symmetrically from the outside-in (Griggs and Choularton 1983). These ice shards grow into needles or columns by vapour deposition to form precipitation, and possibly also more ice-particle-generating graupel. Ice production only occurs at between -3°C and -8°C (with a pronounced peak in the middle of the range) and in the presence of both large (>24 µm) and small droplets. Moderate updrafts help by keeping ice-producing particles within the zone of maximum production.
However, there is conjecture about how the splinters are produced. Dong and Hallett (1989) found that accreting droplets spread out on the ice surface, freezing as cones (frozen droplets formed only at -8°C and below). The rime that grew in their wind tunnel tests was shaped like fingers or staghorn coral. They thus thought that splinters ejected as a result of pressure build-up inside frozen accreted droplets was unlikely.
In general, Phillips et al. (2001) state that “more laboratory work is urgently required in order to establish more quantitatively, accurately and comprehensively than our present knowledge: (1) how the Hallett-Mossop process depends upon temperature, droplet size distribution and velocity of impact; and (2) how many splinters are ejected per freezing raindrop, as a function of drop size and air temperature.”
Indeed a recent and fascinating SEM analysis of graupel and its rime also found no evidence of symmetrical droplets at the appropriate temperatures (Rango et al. 2003). The structure of the graupel suggests a variety of other possible mechanisms, as shown below.











More high resolution photos of graupel and rime can be seen at the Beltsville Agricultural Research Center's Electron Microscopy Unit Snow Page:
http://emu.arsusda.gov/snowsite/rimegraupel/rg.html
http://emu.arsusda.gov/snowsite/FSSP9/FSSP9.html
Mechanisms by which these rime-covered particles might shed ice
1. Large droplets are less deviated by air flow and strike the edge of the graupel. At high temperatures they freeze more slowly (release of latent heat), and backward from the point of contact, as the tail flails in the turbulence. This progressive freezing forms the sinuous, pendulous projections. Due to a combination of lack of deflection leading to collision and release of latent heat slowing the process of freezing, larger droplets break apart, leaving behind a small freezing droplet in the wake of the graupel.
2. Large droplets strike the edge of the graupel, destabilizing its fall and initiating a tumble, causing fragile appendages to snap off.
3. Large droplets embed themselves within the open branch structure of larger graupel, attaching across several projections. When they freeze they expand and break the structure.
4. Branches on the leading face may evaporate as the graupel falls (below -3°C crystals only evaporate, and when evaporating branches thin to <10 µm first; Oraltay and Hallett 1989). Drier air could occur in pockets of the cloud or the cloud mass descends. Or the pressure wave in front of larger graupel cause evaporation (a 500 µm rain drop falling at 4 m/s has a 9 Pa pressure wave in front of it; Chen 2001)?
Bigg, E. K. 1973. Ice nucleus measurements in remote areas. Journal of the Atmospheric Sciences, 30, 1153–1157.
Bower, K. N., Moss, S. J., Johnson, D. W., Choularton, T. W., Latham, J., Brown, P. R. A., Blyth, A. M. and Cardwell, J. 1996. A parameterisation of the ice water content observed in frontal and convective clouds. Quarterly Journal of the Royal Meteorological Society, 122, 1815–1844.
Chen, W. H. 2001. Dynamics of sulfur dioxide absorption in a raindrop falling at terminal velocity. Atmospheric Environment, 35, 4777–4790.
Dong, Y. Y. and Hallett, J. 1989. Droplet accretion during rime growth and the formation of secondary ice crystals. Quarterly Journal of the Royal Meteorological Society, 115, 127–42.
Griggs, D. J. and Choularton, T. W. 1983. Freezing modes of riming droplets with application to ice splinter production. Quarterly Journal of the Royal Meteorological Society, 109, 243–53.
Hallett, J. and Mossop, S. C. 1974. Production of secondary ice particles during the riming process. Nature, 249, 26–28.
Hallett, J., Sax, R. I., Lamb, D. and Ramachandra Murty, A. S. 1978. Aircraft measurements of ice in Florida cumuli. Quarterly Journal of the Royal Meteorological Society, 104, 631–651.
Harris-Hobbs, R. L. and Cooper, W. A. 1987. Field evidence supporting quantitative predictions of secondary ice production rates, Journal of the Atmospheric Sciences, 44, 1071–1082.
Hobbs, P. V. and Rangno, A. L. 1990. Rapid development of ice particle concentrations in small polar maritime cumuliform clouds. Journal of the Atmospheric Sciences, 47, 2710–2722.
Möhler. O., DeMott, P.J., Vali, G. and Levin, Z. 2007. Microbiology and atmospheric processes: the role of biological particles in cloud physics, Biogeosciences, 4, 1059–1071.
Mossop, S. C., Ono, A. and Wishart, E. R. 1970. Ice particles in maritime clouds near Tasmania. Quarterly Journal of the Royal Meteorological Society, 96, 487–508.
Mossop, S.C. and Hallett, J., 1974. Ice crystal concentration in cumulus clouds: influence of the drop spectrum. Science 186, 632–634.
Oraltay, R. G. and Hallett, J. 1989: Evaporation and melting of ice crystals: A laboratory study. Atmospheric research, 24, 169–189.
Phillips, V. T. J., Blyth, A. M., Choularton, T. W., Brown, P. R. A., and Latham, J. 2001. The glaciation of a cumulus cloud over New Mexico, Quarterly Journal of the Royal Meteorological Society, 127, 1513–1534.
Phillips, V. T. J., Choularton, T. W., Illingworth, A. J., Hogan, R. J. and Field, P. R. 2003. Simulations of the glaciation of a frontal mixed-phase cloud with Explicit Microphysics Model. Quarterly Journal of the Royal Meteorological Society, 129, 1351–1371.
Rango, A., Foster, J., Josberger, E. G., Erbe, E. F., Pooley, C and Wergin, W. P. 2003. Rime and graupel: description and characterization as revealed by low-temperature scanning electron microscopy. Scanning, 25, 121–131.
Saunders, C. P. R. and Hosseini, A. S. 2001. A laboratory study of the effect of velocity on Hallett–Mossop ice crystal multiplication. Atmospheric Research 59-60, 3–14.

 Home
Home