The recent publication of Mortazavi et al (2008) made me wonder if we need to be more precise about the meaning of « biological ice nucleation ». This paper points out that a wide range of environmental bacteria can induce freezing of supercooled water above the homogeneous nucleation point of pure water (-39°C). Their work focused on bacteria isolated from snow, and included ubiquitous organisms such as Stenotrophomonas maltophilia, Bacillus spp., and Microbacterium spp. that are also present in a wide range of other habitats. As the authors pointed out, these bacteria induce freezing at moderate temperatures (in the ball park of temperatures where tap water can induce freezing), at about -13°C or colder.
The bacteria studied by Mortazavi et al are much more abundant in the environment than Pseudomonas syringae, Pantoea agglomerans, Xanthomonas campestris pv. translucens and the other bacteria known to express proteins with specific roles in ice nucleation. At first thought, this seems to suggest an even greater potential for the role of biological ice nucleation in cloud processes – at least at intermediate temperatures. But as the authors point out here and elsewhere (Ariya et al, 2008) this intermediate ice nucleation activity is likely due to the presence of various chemicals on bacterial cell surfaces that are inherently ice nucleation active mostly because they provide solid surfaces for nucleation. That observation does not take away from their potential to interact with cloud processes, but it made me wonder if we could determine if this activity was not just something other than that of “dust and debris”.
So I asked the question: can we estimate the added value of biological ice nucleation compared to that conferred by inert chemicals? To do this I tried to find a basis of comparison with one of the most active inert ice nucleators, montmorillonite KSF. Outside of a laboratory this is also known as “French healing clay”. Although it feels rather smooth when I spread it on my face, this substance consists of particles that are hydrophobic platelets (http://www.nanocor.com/nano_struct.asp) that are effective surfaces for initiating the process of ice nucleation. Mortazavi et al report values for the number of ice nuclei per gram of montmorillontie, tested via the immersion freezing droplet test. We have tested the ice nucleation activity of Pseudomonas syringae in the same way, and we can calculate the number of ice nuclei per gram of bacterium. So this could be the basis for a first approach. Admittedly, the chemical components of a bacterial cell are not comparable to montmorillonite. Nevertheless, this comparison allows us to see if biological organization packs a stronger « freezing punch » per gram than a very active but inert substance.
Figure 1 below illustrates the number of nuclei per gram of montmorillonite and of two strains of P. syringae. Data for P. syringae are for strains that represent the range of what we have observed among the hundreds of strains we have characterized. At -4°C for example, strain CC0242 produces one active nucleus per 150 cells and strain CC0170 produces one per 6.3 x 10e7 cells. The rate of nuclei per gram can then be calculated based on the average reported dry weight of bacteria (2.9 x 10e-13 g/cell). For comparison, I also included the data for tap water from Mortazavi et al by assuming that the water contained the maximum acceptable dry residue content for North America, 500 mg/L.
The bacteria studied by Mortazavi et al are much more abundant in the environment than Pseudomonas syringae, Pantoea agglomerans, Xanthomonas campestris pv. translucens and the other bacteria known to express proteins with specific roles in ice nucleation. At first thought, this seems to suggest an even greater potential for the role of biological ice nucleation in cloud processes – at least at intermediate temperatures. But as the authors point out here and elsewhere (Ariya et al, 2008) this intermediate ice nucleation activity is likely due to the presence of various chemicals on bacterial cell surfaces that are inherently ice nucleation active mostly because they provide solid surfaces for nucleation. That observation does not take away from their potential to interact with cloud processes, but it made me wonder if we could determine if this activity was not just something other than that of “dust and debris”.
So I asked the question: can we estimate the added value of biological ice nucleation compared to that conferred by inert chemicals? To do this I tried to find a basis of comparison with one of the most active inert ice nucleators, montmorillonite KSF. Outside of a laboratory this is also known as “French healing clay”. Although it feels rather smooth when I spread it on my face, this substance consists of particles that are hydrophobic platelets (http://www.nanocor.com/nano_struct.asp) that are effective surfaces for initiating the process of ice nucleation. Mortazavi et al report values for the number of ice nuclei per gram of montmorillontie, tested via the immersion freezing droplet test. We have tested the ice nucleation activity of Pseudomonas syringae in the same way, and we can calculate the number of ice nuclei per gram of bacterium. So this could be the basis for a first approach. Admittedly, the chemical components of a bacterial cell are not comparable to montmorillonite. Nevertheless, this comparison allows us to see if biological organization packs a stronger « freezing punch » per gram than a very active but inert substance.
Figure 1 below illustrates the number of nuclei per gram of montmorillonite and of two strains of P. syringae. Data for P. syringae are for strains that represent the range of what we have observed among the hundreds of strains we have characterized. At -4°C for example, strain CC0242 produces one active nucleus per 150 cells and strain CC0170 produces one per 6.3 x 10e7 cells. The rate of nuclei per gram can then be calculated based on the average reported dry weight of bacteria (2.9 x 10e-13 g/cell). For comparison, I also included the data for tap water from Mortazavi et al by assuming that the water contained the maximum acceptable dry residue content for North America, 500 mg/L.
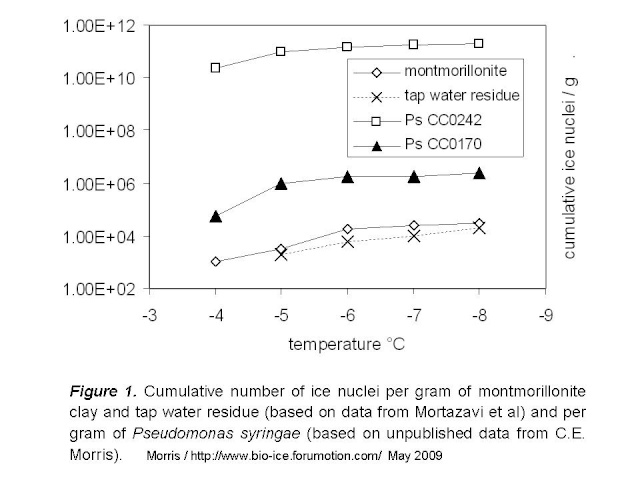
This comparison suggests that the biological organization of chemicals achieved by these strains of P. syringae results in 100-fold to over 10e6-fold higher rates of ice nuclei per gram of matter relative to clay. We should keep in mind that there might be particles of clay (larger particles) that could be more active than what is used in the illustration here, given that particle size and overall surface available for contact with water molecules is decisive for the ice nucleation activity of inert materials. Mortazavi et al did not quantify particle size of clay in these experiments, but it does not seem that they selectively eliminated large particles during preparation of their materials.
Next question: How do the intermediately active strains described by Mortazavi et al compare to strains CC0242 and CC0170 of P. syringae? Quantitative data comparable to the data for clay are not given in the paper, but we can make some good guesses based on the methods they describe. To compare the Mortazavi strains to P. syringae, we can exploit the general pattern of the rate of ice nucleation activity of bacteria, as illustrated in Fig 2, below. These data for strain Cit7 of P. syringae, from which the gene for the ice nucleation protein was first cloned, are from Orser et al (1985). Here I compare them to CC0242 and CC0170.
Next question: How do the intermediately active strains described by Mortazavi et al compare to strains CC0242 and CC0170 of P. syringae? Quantitative data comparable to the data for clay are not given in the paper, but we can make some good guesses based on the methods they describe. To compare the Mortazavi strains to P. syringae, we can exploit the general pattern of the rate of ice nucleation activity of bacteria, as illustrated in Fig 2, below. These data for strain Cit7 of P. syringae, from which the gene for the ice nucleation protein was first cloned, are from Orser et al (1985). Here I compare them to CC0242 and CC0170.
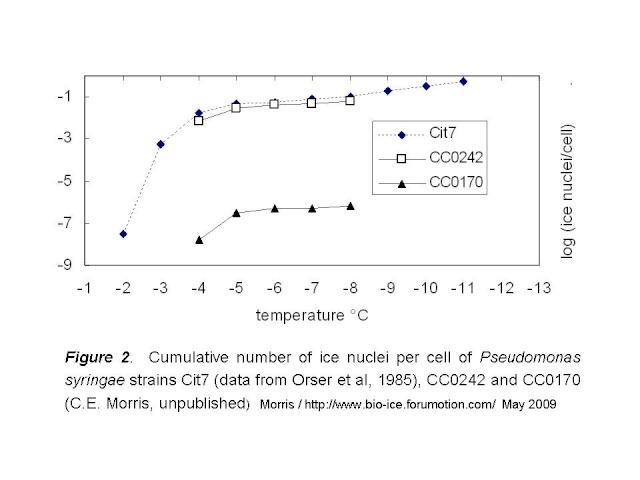
Based on this pattern, I tried to estimate the number of cells that would be active at temperatures colder than -8°C for the least active of the P. syringae strains (CC0170). I used the data for Cit7 to make this estimate. The curve for Cit7 can be readily linearized for the data between -5 and -11°C. The resulting regression has an R2 of 99%:
(log (ice nuclei / cell))2 = 0.973 x ΔT°C
The predicted values for rate of ice nucleation activity for strain CC0170 are given by the red curve in Fig 3 below.
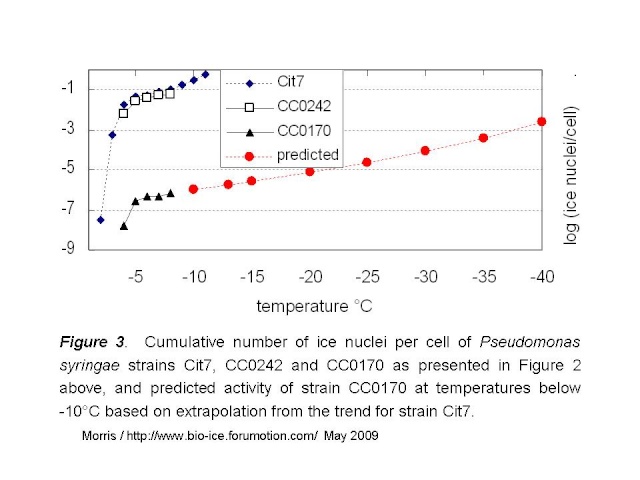
Mortazavi et al tested bacterial suspensions adjusted to 10e8 cells/mL. If we assume that they tested 216 droplets at 5µL / droplet, as they did for the freezing tests of clay, then they would have tested a full mL of suspension and thus about 10e8 cells. Given the standard deviation of the reported mean freezing temperatures for their strains (the warmest being -12.9°C +/- 1.3), it would be reasonable to guess that no droplets froze at temperatures warmer than -10°C. Hence, these intermediately active strains would not have more than 1 ice nucleus per 10e8 cells at -10°C. If the relationship between temperature and rate of ice nucleation active cells for the intermediately-active strains is similar to that of P. syringae, then the maximum activity for intermediately active strains could be represented by the green line in Figure 4 below.
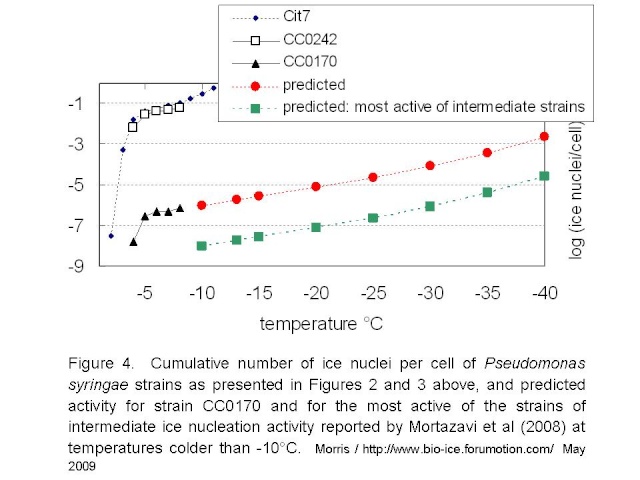
These estimates lead me to suggest the following. Firstly, if the rate of ice nucleation activity of the intermediate strains is 2 orders of magnitude below that of strain CC0170 of P. syringae, then the activity of these intermediate strains is similar to that of montmorillonite clay or tap water residue. Hence, it raises the question of the importance of biology per se in this activity. Clearly these bacteria are biological entities. But is their ice nucleation activity not simply due to accumulation of chemicals on rather large particles? It appears as if there are no selective forces of nature that have pushed this activity to an optimum efficiency - in stark contrast with the case for the highly active strains of P. syringae. Secondly, a rather high number of cells are needed to produce nuclei active near the homogenous nucleation temperature of water (ca. -40°C): about 1000 cells for the weakest of the P. syringae strains and about 10e5 cells for the strains with intermediate activity. This makes me think about the results reported by Junge and Swanson (2008) indicating an absence of ice nucleation activity above -40°C in a range of gamma-Proteobacteria strains. Is there something distinct about their strains that would make them recalcitrant to freezing even as chemical surfaces? For each strain, Junge and Swanson tested the freezing of 1000 droplets each containing 3-5 cells. Hence, they tested 3000 to 5000 cells. I can’t resist wondering about the results had they tested 10e8 cells.
This intellectual exercise brings out the need for comparable data among the different ice nucleators for which we hope to estimate their role in atmospheric processes. It also suggests that for the highly active biological ice nucleators, the evolutionary forces that mold protein functions have been very effective in honing the efficiency of these proteins as ice nucleators.
Literature cited
Ariya P.A., Sun J., Eltouny N.A., Hudson E.D., Hayes C.T. Kos G. 2009. Physical and chemical characterization of bioaerosols - Implications for nucleation processes. International Reviews in Physical Chemistry 28:1-32
Junge K., Swanson B.D. 2008. High-resolution ice nucleation spectra of sea-ice bacteria: implications for cloud formation and life in frozen environments. Biogeosciences 5:865–873.
Mortazavi R., Hayes C.T., Ariya P.A. 2008. Ice nucleation activity of bacteria isolated from snow compared with organic and inorganic substrates. Environ. Chem. 5, 373–381.
Orser C., Staskawicz B.J., Panopoulos N.J., Dahlbeck D., Lindow S.E. 1985. Cloning and expression of bacterial ice nucleation genes in Escherichia coli. J. Bacteriol. 184:359-366.
This intellectual exercise brings out the need for comparable data among the different ice nucleators for which we hope to estimate their role in atmospheric processes. It also suggests that for the highly active biological ice nucleators, the evolutionary forces that mold protein functions have been very effective in honing the efficiency of these proteins as ice nucleators.
Looking forward to discussion about this subject.
Cindy Morris
21 May 2009
Cindy Morris
21 May 2009
Literature cited
Ariya P.A., Sun J., Eltouny N.A., Hudson E.D., Hayes C.T. Kos G. 2009. Physical and chemical characterization of bioaerosols - Implications for nucleation processes. International Reviews in Physical Chemistry 28:1-32
Junge K., Swanson B.D. 2008. High-resolution ice nucleation spectra of sea-ice bacteria: implications for cloud formation and life in frozen environments. Biogeosciences 5:865–873.
Mortazavi R., Hayes C.T., Ariya P.A. 2008. Ice nucleation activity of bacteria isolated from snow compared with organic and inorganic substrates. Environ. Chem. 5, 373–381.
Orser C., Staskawicz B.J., Panopoulos N.J., Dahlbeck D., Lindow S.E. 1985. Cloning and expression of bacterial ice nucleation genes in Escherichia coli. J. Bacteriol. 184:359-366.

 Home
Home